方案
By Technology
公司介绍
十一月 13, 2023
Share:

The Over the Counter (OTC) Hearing Aid Act passed US legislation in 2017 requiring the Food and Drug Administration (FDA) to propose a rule that would establish an OTC hearing aid category for adults with “perceived” mild to moderate hearing loss. On August 17th, 2022 the FDA finally released its official ruling on this legislation, publishing a lengthy document including thorough and well-reasoned responses to the most important comments. It’s been one year since the FDA’s official ruling so I thought it would be useful to look back at the history of how we got here, how the landscape has changed over the past year and what the future might look like for OTC hearing aids.
The overarching goal of the OTC legislation and subsequent FDA ruling is to increase the availability and affordability of hearing aids for adults with perceived mild to moderate hearing loss. Perceived hearing loss means that it is the patient themself who believes they have a mild to moderate hearing loss and there is no requirement for the hearing loss to be diagnosed or measured by a professional. The final FDA rule and its responses to comments make it clear that OTC hearing aids are truly “over the counter” and should not require the participation of a licensed professional (audiologist) in any way. While seeing a licensed professional is certainly not discouraged (it has many obvious benefits), it is not required.

In general, the reaction to the FDA ruling has been overwhelmingly positive; from the device manufacturers to the hearing health professionals. Since four-fifths of the potential market goes unserved and people generally wait 5-7 years from the onset of hearing loss until they seek professional help, it is felt that the OTC ruling will not only encourage people to attempt to help themselves earlier but that it will also serve as a gateway to seek professional help earlier. This may grow the current customer base served by audiologists, rather than erode it. Manufacturers also see the opportunity to market a new crop of devices that are simpler and potentially at a lower price point, with the idea that users will eventually “trade up” to more functional devices as they age, earning some brand loyalty along the way. Jabra, in particular, is releasing its Enhance Plus earbuds as an OTC hearing aid and the average age of an Enhance Plus user is 18 years younger than the average prescription hearing aid user.
So how have things unfolded in the marketplace over the past year? Well, the consensus seems to be that it has yet to make the desired impact as shown by a recent survey from the American Speech-Language-Hearing Association (ASHA). While hearing aids are more accessible and slightly more affordable (a professionally fit set of clinical hearing aids can cost many thousands of dollars, while a good pair of OTCs are generally between $800-$1200), they can still be difficult to use and are still relatively expensive for something bought “over the counter”. According to a recent article, “OTC aids made up less than 1% of traditional hearing aid manufacturers’ sales in the first quarter of 2023”, a number that was expected to be bigger given that there are only ~12,000 audiologists in the US servicing a potential market of 45 million people, and most Americans live within 5 miles of a pharmacy, Best Buy or Walmart where OTC hearing aids are now sold.
According to the survey the biggest reason people reported not seeking professional help for a hearing loss problem continues to be the belief that their hearing loss simply wasn’t bad enough to warrant a hearing aid. While the perceived lower cost and accessibility of OTCs certainly lowers the barrier to entry, the first step to treatment is acknowledging you have a problem.
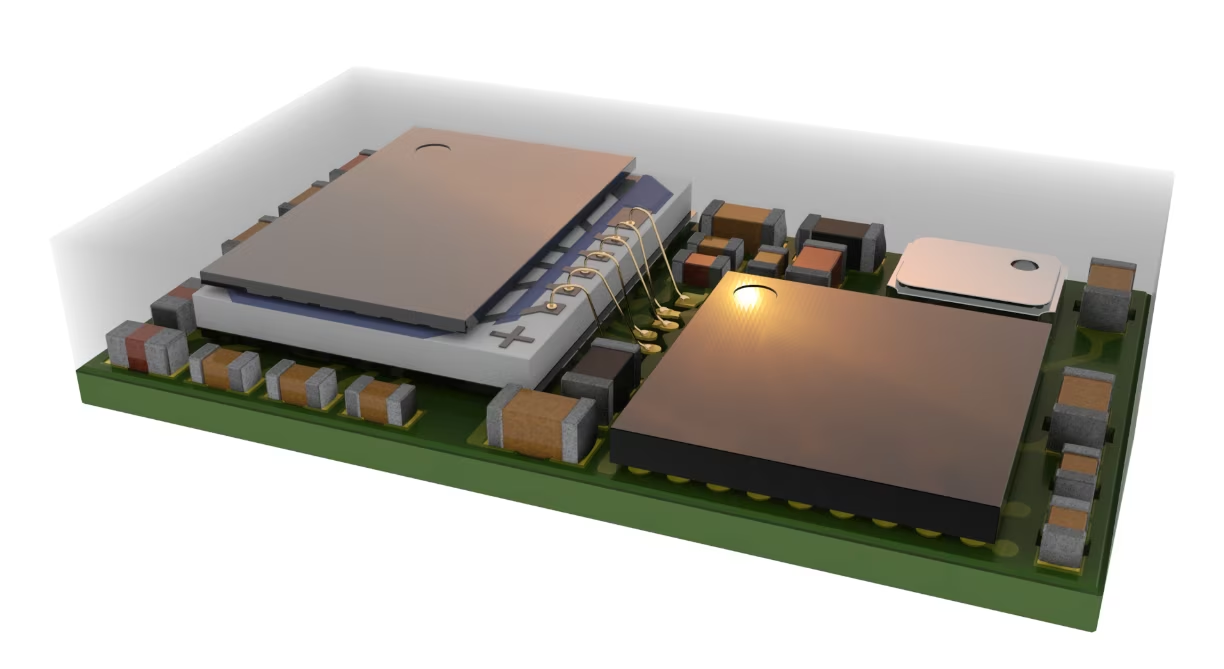
The Ezairo 7160 SL audio processor from onsemi, combined with its Ezairo Pre Suite firmware and software provides a powerful solution for both clinical and OTC hearing aids. Its exceptional audio quality and low power wireless technology allow manufacturers to design and build cutting edge devices that offer real solutions for people with hearing loss. As the OTC market continues to evolve and access to hearing aids broadens, onsemi will continue to innovate and provide the solutions device manufacturers need to address this growing market.
Hearing loss is a silent problem that often goes untreated for years. Recent studies have shown that adults with untreated hearing loss have significantly higher rates of developing psychosocial disorders such as depression, anxiety, and dementia and incur higher medical bills than those without hearing loss. Today’s hearing solutions include removable and implantable hearing aids such as advanced digital hearing aids and cochlear implants for those with severe hearing loss, respectively.